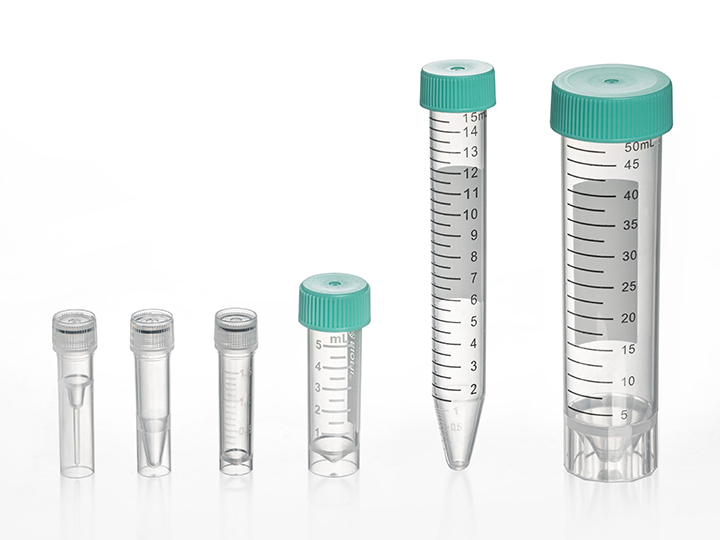
* What is the maximum rotational speed that the centrifuge tubes of Jet Biofil can withstand?
21000xg. Due to the different center distance of the rotor shaft of different centrifuges, the centrifugal force generated at the same speed (rpm) is also different, so we usually use the relative centrifugal force (RCF) (g) to represent the tolerance degree of the centrifuge tube in the process of rotating centrifugation.
* Can the centrifuge tubes of Jet Biofil hold some kind of solution? How about its chemical compatibility?
It is necessary to pay attention to whether the sample is compatible with the material of the centrifuge tube as is used, especially if the sample is some chemical reagents or organic solvents, and the Chemical Compatibility Table of PP Plastic Materials can be used as reference. Samples with low or incompatible centrifugal compatibility are not recommended, and customers can also do pre-experiments to have a test.
* What is the possible reason for the burst of the centrifuge tubes in the process of centrifuge?
When centrifuging, it is necessary to pay attention to the chosen centrifugal force cannot exceed the maximum centrifugal force that the product itself can withstand.
Please notice that the maximum centrifugal force (RCF) that can be withstood may be decreased when the sample is centrifuged at low temperature or the sample is an organic solvent/volatile irritant.
* What should be pay attention to when using the centrifuge tubes to preserve the samples at a low temperature?
When the sample is preserved below 0 ℃, the applicability of the centrifuge tube is closely related to the composition of the solution and preservation conditions. The centrifuge tube cannot be placed on the foam support, nor the bottom of the tube is in contact with the thermal insulation materials. And please use a hollow support when preserving the samples at low temperature. Otherwise, as part of the centrifuge tube is protected by thermal insulation material, while the other part is not, the temperature difference of the overall tube body makes the tube prone to burst. In addition, please reserve a space of at least 10% of the total volume to avoid sample spillage or tube burst caused by volume expansion due to liquid solidification.
* Why the centrifuge tube smell special when its package is opened?
If you smell some odor in the product you receive, it is not because of the abnormal quality of the product. This is because some molecules will be vaporized and retained in the product/packaging in the process of high temperature injection molding/irradiation sterilization of the raw material plastic particles of centrifuge tube products.
The materials used for making the centrifuge tubes of Jet Biofil comply to the USP Class VI standards (USP Class VI Chapter<87>, "Biological reactivity Tests, in Vitro" and Chapter<88>, "Biological Reactivity Tests, in vivo”). No cytolysis or cytotoxic effects were observed through the verification. And the outgoing products in the process of quality inspection have passed various verification tests and are in line with the product release standards.